About CRU
Clinical research forms the cornerstone of evidence-based medicine and advances in clinical practice. An essential component of clinical research is clinical trials, some of which may be multi-centric and engage collaborative efforts. Non-trial clinical research is the other equally important component which helps to understand and answer critical treatment-related questions, identify further research questions, and support development of new biomarkers, etc. There is a need for clinical research designed and led by academic Investigators to address the specific challenges that patients face in India.
Researchers at AIIMS come with varied scientific backgrounds and levels of exposure. While most are well conversant with the intricacies of research methods, a few may need guidance to develop their idea into a research protocol or to arrange funding or with conduct, analysis, and publication of the study. Also, there is a need to conduct larger studies and trials to make a meaningful impact on the care of patients. In addition, excellent data management and statistical analytical skills are required for optimal utilization of the data generated in various clinical studies.
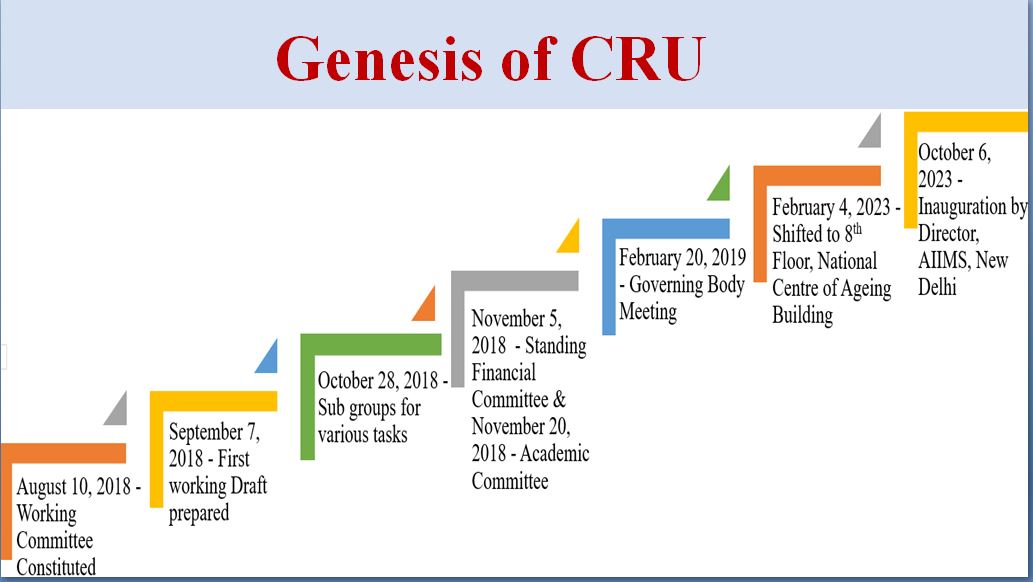
The Honourable Vice President Sh. Venkaiah Naidu in his address on 7 Dec 2018 on the occasion of 46th Convocation Day of AIIMS, stressed upon the need for the institute faculty to take clinical research to a higher level and to set up newer mechanisms to foster collaborative research answering translational research issues. The Honourable Vice President urged the AIIMS administration and faculty to launch new initiatives which facilitate clinical research. The administration under the leadership of the honourable Director of AIIMS, Prof. Randeep Guleria and the Dean (Research), Prof. Chitra Sarkar and with the guidance and sage advice of the former Secretary, Department of Biotechnology (DBT) Late. Prof M.K. Bhan conceptualized and created the Clinical Research Unit (CRU) under the Research Section, AIIMS, New Delhi.
The Clinical Research Unit shall serve the purpose of improving the quality and quantum of research output through i) providing high-quality technical expertise in designing clinical research, and ii) providing support for the conduct of studies conforming to international standards.